Case Study: Global Pricing Strategy for a New Vaccine
Project Objective
A global pharmaceutical company was planning to launch a next generation vaccine into a competitive market with the aim of replacing the current standard of care, which was a vaccine that they also owned.
The market will be very competitive at launch because a competitor is also developing a next-generation vaccine, that is expected to launch in advance of our client.
Windrose was asked to evaluate the price and market access opportunities and challenges for the client’s vaccine, and to aid the development of a global pricing and reimbursement strategy to inform forecasting. We were also asked to develop strategies to defend their current vaccine against competitors.
Windrose Approach
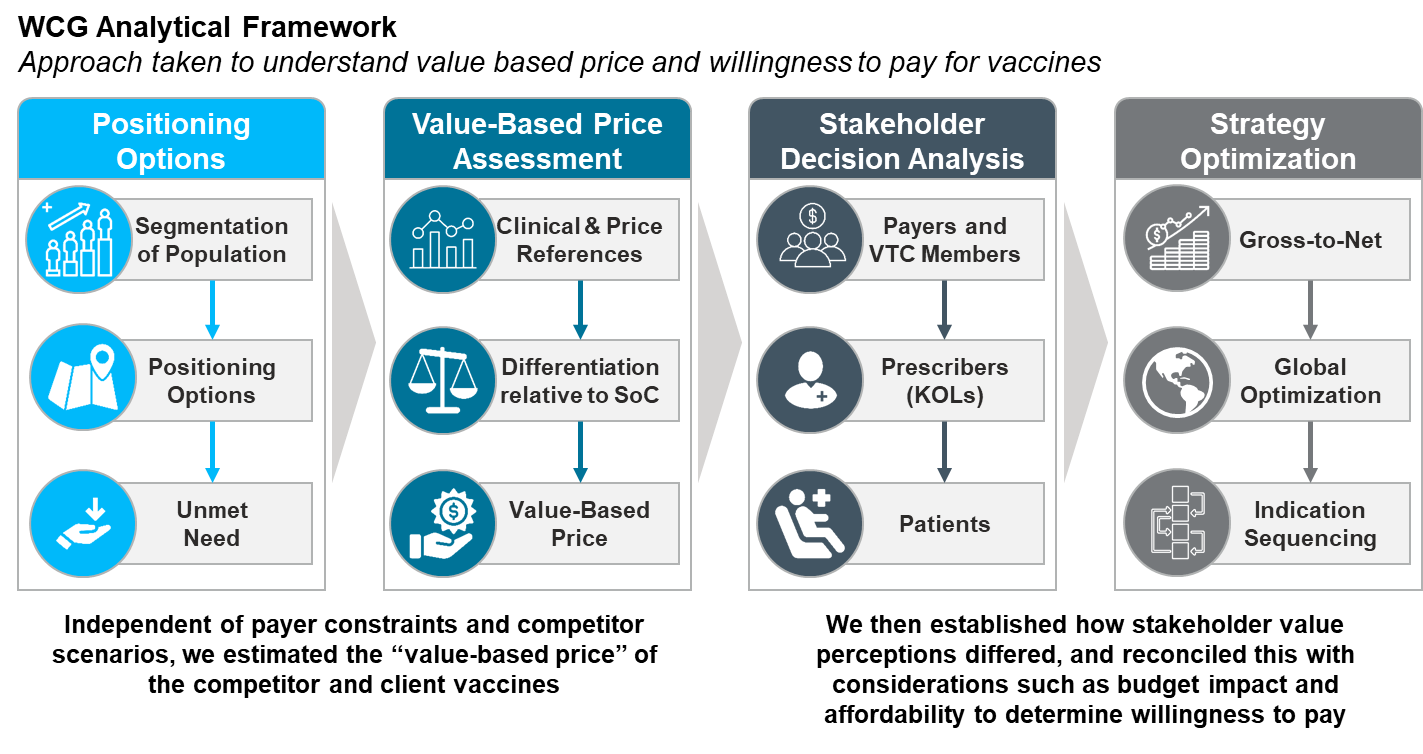
Windrose Consulting Group Analytical Framework
We worked with global and local teams to analyze the vaccine pricing and market access landscape for 13 markets across the US, Europe, Latin America, Asia and the Middle East.
We conducted primary payer, key opinion leader and vaccine technical committee research across markets to gauge stakeholder perceptions of both the competitor and the client’s vaccine, as well as the vaccine market access landscape.
We also assessed several competitive scenarios with stakeholders to help our client evaluate the price-access tradeoffs that may be required as each vaccine entered the market.
Impact
We developed a global pricing and reimbursement strategy to support product launch across markets; this prepared the client for entry into a competitive market helping to maximize its market share, revenue, and reimbursement status globally.
We also identified the steps the client needed to take to prepare for launch, including the development of payer value propositions, pricing defense and revenue optimization strategies.
The final recommendations were well received by both the global and local teams and were used as a foundation for the development of the global pricing policy

