Case Study: Pre-Clinical Landscape Assessment
Landscape assessment for a product in pre-clinical development for Parkinson’s Disease
Project Objective
A global pharmaceutical company was developing a new product for the treatment of Parkinson’s disease; the product had a novel mechanism of action and was in early phase of clinical development
At the time of development, the Parkinson’s Disease market was saturated; therefore, the client’s product needed to demonstrate meaningful differentiation across current and future competitors to secure favorable price and market access
Windrose was asked to evaluate the P&MA opportunities and challenges for the client’s product to inform further investment and clinical development decisions, and also to identify core evidence requirements and strategies that could minimize market access barriers in the future
Windrose Approach
We worked with the cross-functional client team to identify potential positioning options and evidence strategies, as well as developing hypotheses related to each project objective
We conducted primary market research with national and regional payers and key opinion leaders in the US and EU3 to understand the disease landscape, and the P&MA potential of the product
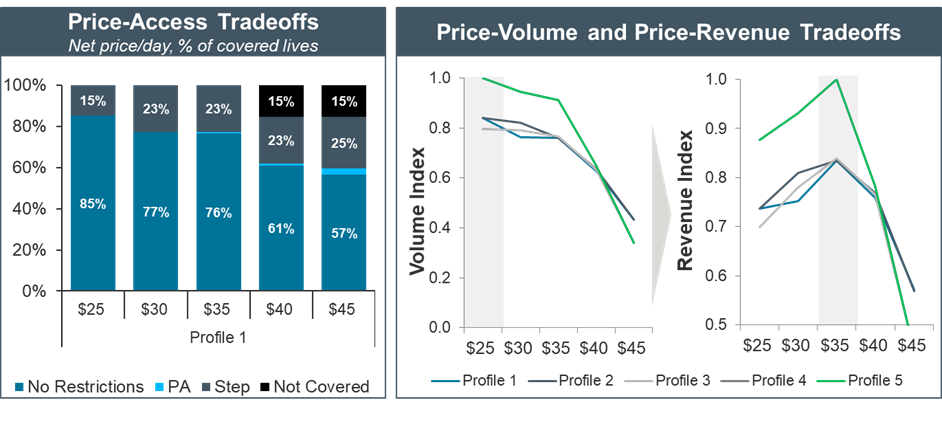
As the product was in the early stages of development, we tested five different profiles with stakeholders to understand how different trial designs and clinical profiles could affect the P&MA potential in the future
This involved an in-depth analysis of the HTA outcomes, price references, access-potential, and price-access trade-offs for each of the five product profiles
Impact
We identified the key value drivers and barriers for the product, and the evidence required to optimize its P&MA potential across different markets
We provided recommendations on how to optimize the revenue of the product, including; offering different levels of rebate to increase access; identifying price and utilization trade-offs to overcome payer restrictions and patient co-pays; and evaluating how competitor strategies, such as discounting, could impact utilization
Our recommendations were used by the client to inform further clinical decisions for the product, and to aide the design of future clinical trials and evidence generation strategies

