France Early Access Program Changes
The Old Process – ATU and RTU
Established in 1992, the Authorization for Temporary Use program in France (ATU) is among one of the oldest and most open early access programs in Europe.[1] It allows patients with rare or serious diseases access to drugs which are currently unauthorized in that indication. Although a complex process, it appeals to manufacturers, with recent funding for ATUs growing to over €1 billion a year.[2]
France was the first country in Europe to establish a national framework for approved use of off-label drugs. The Recommendation for Temporary Use (RTU) was introduced in 2012 for use of off-label drugs where there was an unmet need and a favorable risk/benefit ratio. Exceptionally, RTU could be granted based on economic ground, as was the case for Avastin in wet macular degeneration.[3]
The Change – Early Access and Compassionate Access
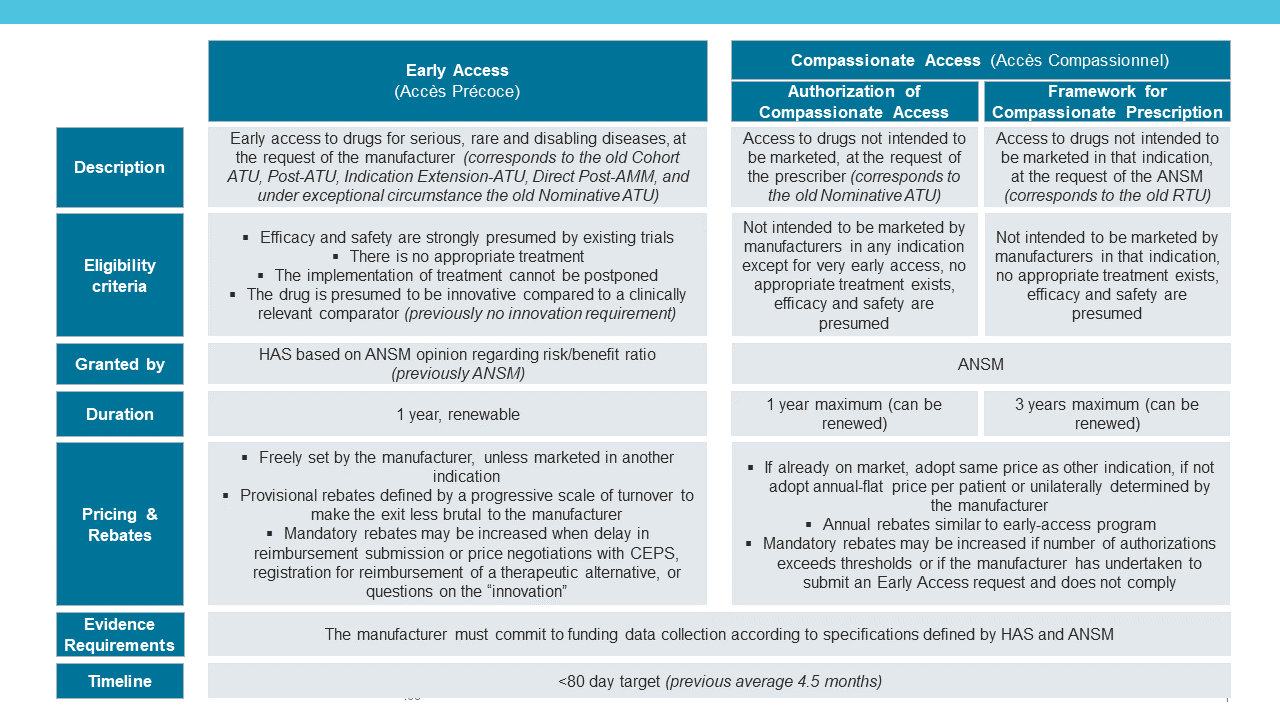
Over time, the ATU/RTU mechanisms have become more complex with the evolvement of different streams. This prompted a review by the French Social Security Law for 2021 (Loi de financement de la Sécurité sociale (LFSS) pour 2021 [4]) to simplify the system, to become more homogenous and to obtain a more financially sustainable solution. The ATU/RTU program, previously divided into 6 streams, will now become 2: Early Access (Accès Précoce) and Compassionate Access (Accès Compassionnel).
The main details are listed in the table however, to summarize the key changes [2,5,6,7]:
Early Access, which replaces most old ATU mechanisms (including the nominative ATU under exceptional circumstances), now also includes “disabling” diseases, and products are subject to additional eligibility criteria: the drug must be deemed “innovative” compared to a relevant comparator.
The presumption of “innovation” includes: a novel treatment, likely to be a substantial or major contribution to the evolution of care in France, a minimum level of clinical data depending on the stage of development, and few uncertainties in tolerance.
Compassionate Access, which replaces the old RTU and the nominative ATU mechanisms, now only considers drugs where further launch development in that indication is not planned, and the new law does not mention the possibility of an off-label use based on economic reasons.
For both pathways, rebates previously calculated at the end of the program are now “provisional”, calculated on a progressive scale of turnover to minimize the impact for the manufacturer when they exit the program and so that the budget can be appropriately managed.
In addition, mandatory rebates for the Early Access may be increased if there is a delay in reimbursement submission or CEPS price negotiation, questioning of the products’ “innovation”, or registration for reimbursement of a therapeutic alternative.
Similarly, mandatory rebates for the Compassionate Access may be increased if the manufacturer has undertaken to submit an Early Access request and does not comply, or when the number of Compassionate Access authorizations exceeds the agreed threshold.
TABLE 1: OVERVIEW OF THE FRENCH EARLY ACCESS PROGRAM CHANGES
Windrose’s Take
Despite the noise surrounding the change, there could be minimal implications for manufacturers. The attractiveness of the early access program in France has not diminished, since manufacturers are still able to set free pricing which indirectly becomes a price ceiling for CEPS negotiations. The innovation criteria could refine the number of eligible treatments for Early Access compared to the old system to those more promising from a Health Technology Assessment (HTA) perspective.
The more limited application for compassionate use of off-label drugs in patients will help avoid controversial use and the goal to shorten the process to 80 days should result in fewer administrative delays. The defined requirements for demonstrating real-world evidence for patients during the program provide clarity for manufacturers, but this may disadvantage smaller companies who do not have a local presence in France.
While the new regime seems to simplify the past ATU/RTU mechanisms, some uncertainties exist, and it remains to be seen how the Early and Compassionate Access pathways will be implemented.
This article was co-written by Georgina Powell, Analyst and Anaïs Frappé, Principal at Windrose Consulting Group.